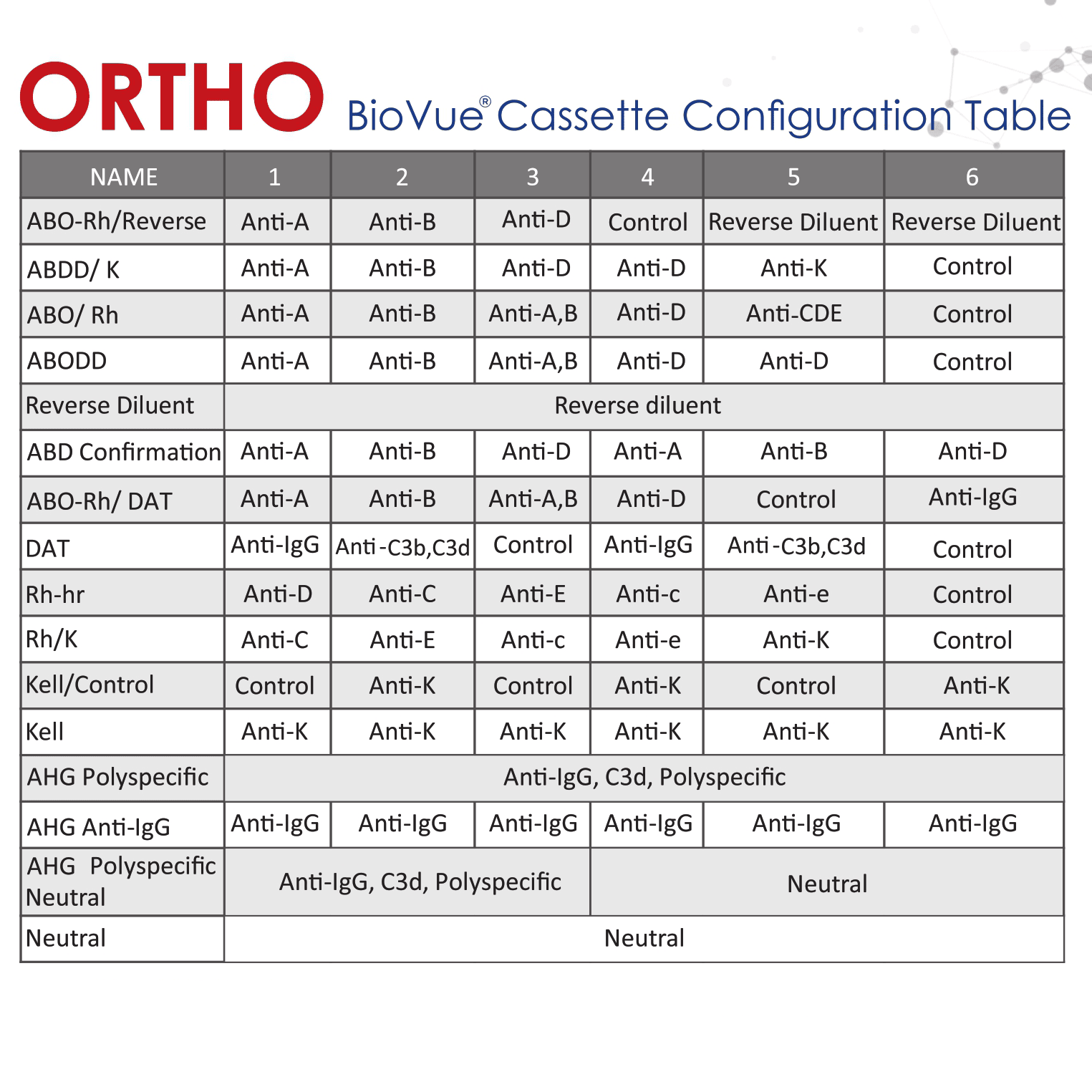
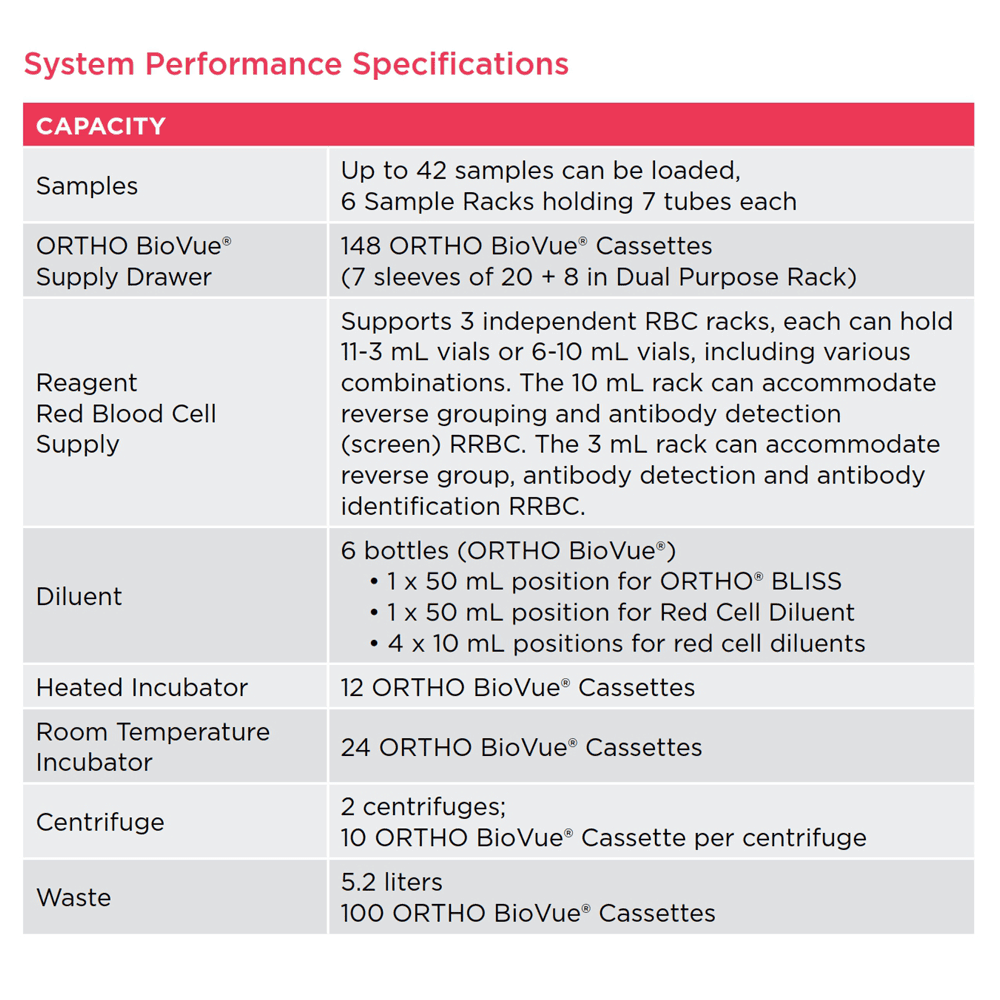
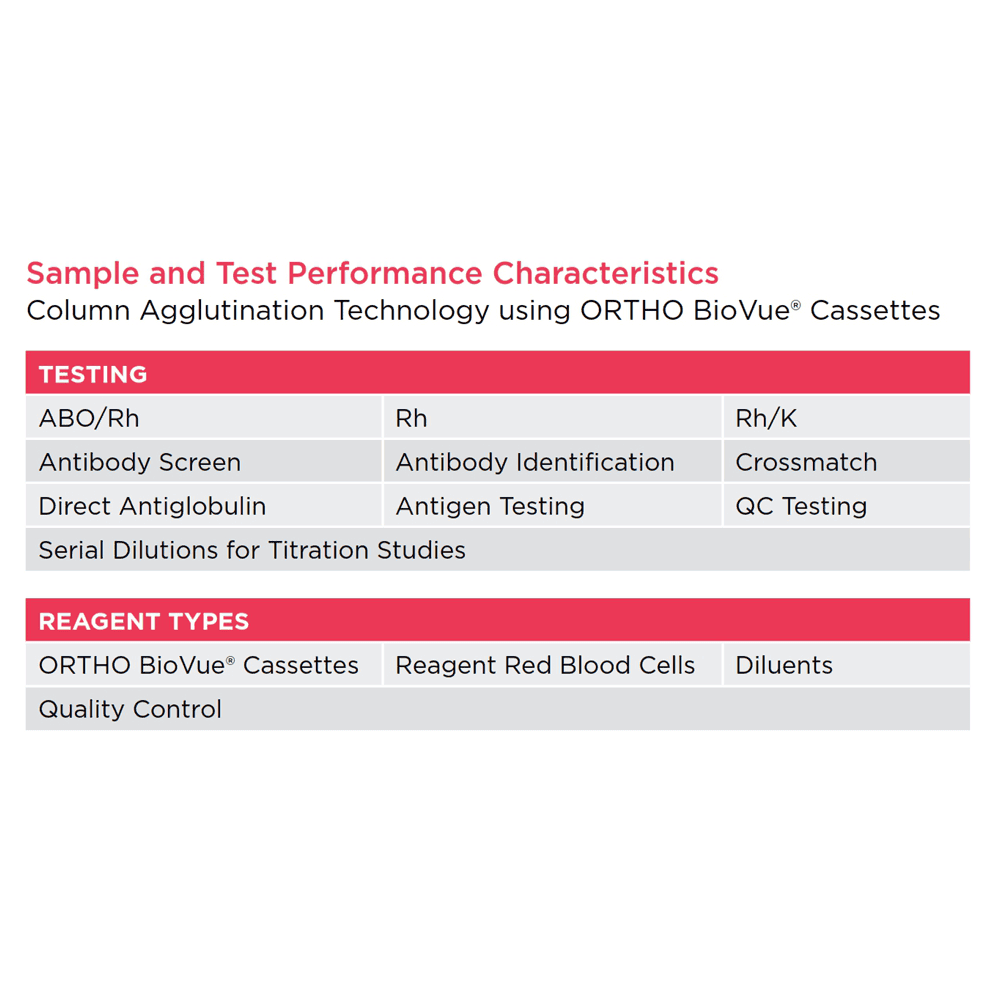
ORTHO VISION™ Analyzer:
• Automates test processing functions including liquid pipetting, reagent handling, incubation, centrifugation, reaction grading and interpretation and data management requirements using ORTHO BioVue® Cassettes and digital image processing.
• Standalone instrument or interfaced to the Laboratory Information System (LIS).
• Monitors critical processing monitoring and provides for complete management of system maintenance and quality control.
Installation and Site Specifications
Although trained service personnel install the ORTHO VISION™ Analyzer at the laboratory site, the site must be prepared according to site specifications.
Physical Dimensions
ORTHO VISION™ Analyzer
Width: 107.4 cm (42.3 in.)
Depth: 77 cm (30.31 in.)
Height: 88.9 cm (35 in.)
Height with maintenance door open: 137 cm (54 in.)
ORTHO VISION™ Table Dimensions (highly recommended)
Table without shelf:
121.9 cm (48 in.) x 76.2 cm (30 in.) x 76.2 cm (30 in.)
Table with shelf at the front:
121.9 cm (48 in.) x 106.7 cm (42 in.) x 76.2 cm (30 in.)
- Occupies 61 cm (24 in.) on left side front of table width
Table with the shelf on the side:
152.4 cm (60 in.) x 76.2 cm (30 in.) x 76.2 cm (30 in.)
- Occupies 61 cm (24 in.) in depth on left side – side of table
Weights
ORTHO VISION™ Analyzer: 192 Kg (424 lbs)
ORTHO VISION™ Table: 120.2 kg (265 lbs)
Power Requirements
One dedicated (3-wired single phase; line-neutral and single circuit) AC power line for connection to facility power.
Input Voltage 100 - 240 V AC 50/60 Hz 1000 VA
Environmental Specifications
• Operating Temperature: 18 - 30° C (64.4 - 86.0° F)
• Site Relative Humidity: 15 - 85% RH (non-condensing)
• Maximum Altitude: 2438.0 m (8000 ft.)
• Heat Output: 3412 BTU/hr
e-Connectivity® Remote Connectivity
Provides the ability to connect your system to Ortho Clinical Diagnostics in a way that enables remote diagnostics as well as, monitor and review system configuration, data, and performance.
e-Connectivity® Technology Ready
Network Connection Requirements:
• Category 5e cable with male RJ45 connector for SSL connection included.
• Continuous broadband connection or direct connection to the site LAN with access to the Internet at a speed greater than or equal to 128 kbps.
• Support local area network port speeds of automatic, 10/100 Mbps with half and full duplex, and 1 Gb full duplex and automatic detection of duplex.
• Dynamic or Static IP Address, Subnet Mask and Default Gateway IP Address assigned by the IT department and provided to OCD.
• Female RJ45 connector on network port within 20 feet of system center.
Broadband Internet
A broadband Internet connection is required
Supported Sample Types
• Centrifuged whole blood
• Plasma and Serum
• Packed red blood cells
Supported Sample Tube Sizes
• 16 x 100 mm, 16 x 75 mm
• 12-13 x 100 mm, 12-13 x 75 mm
• 10.25 x 75 mm, 10.25 x 64 mm
• 15 x 92 mm Sarstedt 01.1605.100
• 13 x 90 mm Sarstedt 04.1931.100
• 2.0 mL and 1.5 mL micro-collection containers
• Wide variety of Pediatric Tubes
Sample and Test Processing
Continuous, random, STAT access and batch
System Computer and Interface Specifications
Interface Specification: Bidirectional protocols for a Laboratory Information System (LIS).
Remote Review Capable: An external computer on the laboratory’s network where Authorized personnel on an external computer can review results.
LIS Specifications
LIS interface via one of three user configurable physical interfaces:
1. ASTM over RS-232
2. ASTM over TCP/IP
3. Network shared folders
(similar to ORTHO® AutoVue® Innova System)
ASTM protocol is configurable to one of three options:
1. Basic ASTM (no ‘M’ record)
2. Enhanced ASTM (very similar/backward compatible to AutoVue Innova)
3. Vision ASTM – which adds in addition to Enhanced ASTM:
a. Error upload message if the order could not be processed.
b. The LIS can download multi-tube orders.
c. The LIS can send QC orders that specify the cassette/ reagent lots to use as well as the expected results.
Communication Ports
I/O ports include:
• 1 DB-9 serial port
(RS-232 port for LIS support)
• 1 RJ45 LAN port supports
port speeds of automatic, 10/100 Mbps with half and full duplex, and 1 GB full duplex and automatic detection of duplex. 5 V 2.0/V 1.1 USB ports are
available for:
• Printer
• Handheld barcode reader
• Other devices
Printer Specifications
ORTHO VISION™ Analyzer can be connected either to a network printer or to a local printer.
Description
ORTHO VISION™ Analyzer automates in vitro Immunohematology testing of human blood utilizing ORTHO BioVue® Cassette technology.